Abstract
Background: The advent of Tyrosine Kinase Inhibitors (TKI) totally changed the outcome of patients affected by Chronic Myeloid Leukemia (CML). Most of informations about the clinical characteristics of CML patients are based on data derived from clinical trials, that often exclude patients not fitting with strict inclusion criteria. In real life may be important to know the characteristics of the entire CML patients' population and this is better reflected by registries studies including all consecutive patients.
Aim: To provide a robust and updated information on clinical, hematologic characteristics and treatment response in non-selected Italian patients with CML in each phase of the disease.
Methods:We retrospectively and prospectively recorder in a web-based database clinical, hematological, cytogenetic and biological informations about all new diagnosis of CML patients referred to Italian Hematology Centers of the GIMEMA Study Group from January 2012 to June 2016.
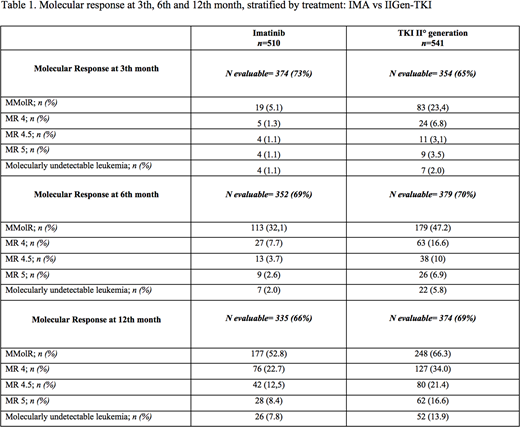
Results:Our study covered 1051 patients newly diagnosed with CML and treated between January 2012 and June 2016 in 66 Italian centers. Median age at diagnosis was 59 years (range 47-71) and 60.8% were males. At diagnosis among 908 patients 899 (99%) patients were in chronic phase, 7 (0.8%) patients in accelerate phase and 2 (0.2%) in blastic crisis. Among 1051 patients, 148 (14%), 351 (33%), 318 (30.3%) were high, intermediate and low risk by Sokal with 234 (22.2%) missing; 54 (5%), 418 (40%), 342 (32.5%) high, intermediate and low risk by EURO with 237 (22.6%) missing; 55 (5%) and 797 (76%) high and low risk by EUTOS with 199 (18.9%) missing; 96 (9%), 208 (20%), 533 (51%) high, intermediate and low risk by ELTS with 214 (20.4%) missing, respectively. The median follow-up was 36 months and 36 patients died (10 CML related). At cytogenetic analysis, there were 887 available informations: 809 (77%) without additional cytogenetic aberrations (ACA), 49 (5%) with major route and 29 (2.8%) with minor route ACA respectively. BCR-ABL transcripts among 873 data available were: b2a2 in 303 (34.7%), b3a2 in 493 (56.5%), b2a2+b3a2 in 61 (7%), e1a2 in 12 (1.4%), e19a2 in 2 (0.2%) b3a2+e1a2 in 2 (0.2%) patients respectively. ECOG performance status among 801 patients was 0, 1, or ≥ 2 in 585 (73%), 181 (22.6%) and 35 (4.4%) cases respectively. According to co-morbidity and excluding CML diagnosis as parameter, among 995 cases the Charlson comorbidity index, was 0, 1, 2 or ≥3 in 771 (73.4%), 115 (11%), 60 (5.7%) and 49 (4.7%) patients respectively; among 556 patients cardiovascular, lung, metabolic and oncologic diseases were observed in 283 (24%), 108 (10.3%), 77 (7.3%) and 88 (8.4%) patients respectively. Five hundred-ten (48.5%) and 541 (51.4%) patients were treated in first-line with imatinib (IMA) and with II generation TKI (IIGen-TKI) respectively. Three hundred twenty-one (30%) cases were pretreated with hydroxyurea. Molecular responses data at 3th month were available in 728 patients and of these 102 (14.01%) obtained at least Major Molecular Response IS (MR) MR3, 29 (3.98%) MR4, 27 (3.71%) MR4.5, 13 (1.79%) MR5 and 11 (1.5%) undetectable BCR-ABL1transcript, respectively. At 6thmonth, among 731 data available, at least 292 patients (39,57%) obtained MR3, 90 (12,19%) MR4, 75(10.16%) MR4.5, 35 (4.74%) MR5 and 29 (3.97%) undetectable BCR-ABL1transcript, respectively. At 12thmonth among 709 molecular responses available, 425 patients (59.94%) achieved at least MR3, 203 (28,63%) MR4, 171 (24.12%) MR4.5, 90 (12.69%) MR5 and 78 (11%) undetectable BCR-ABL1transcript, respectively. As showed in table 1, the IIGen-TKI were able to obtain a higher, earlier and deeper molecular response at 3th, 6thand 12thmonth than IMA. Data analysis about responses at 24thmonth are ongoing. Overall survival at 5 years was 93.4% (95% IC 90.1-95.6).
Conclusions:Our preliminary results of this observational epidemiologic study suggest that collection of clinical data of CML patients treated out of strictly clinical trials represent an essential tool for long/term treatment, able to observe setting strategies based on the clinical characteristics, the degree of response obtained, and the toxicity related to the therapy in overall CML population. We are planning to continue to analyze all these endpoints to estimate the response and toxicity according to ELN guidelines, and feasibility of treatment sequence in a cohort of patients treated in real-life.
Castagnetti:Pfizer: Consultancy, Honoraria; Bristol Meyers Squibb: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Incyte: Consultancy, Honoraria. Breccia:BMS: Honoraria; Incyte: Honoraria; Pfizer: Honoraria; Novartis: Honoraria. Pane:AMGEN: Speakers Bureau; Novartis: Research Funding, Speakers Bureau; BMS: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal